Collecting detailed microscopic images is just one part of the experimental process for WID’s BIONATES research group, where scientists and students create tissue for studying disease as well as engineer solutions to health and industrial problems.
But when researchers examine these images, they’re often in awe of what they see — colorful fluorescent landscapes, artful strokes, and a multitude of cell shapes and signals.

Max Salick, an engineering graduate student who works in BIONATES, produces these micro-sized glimpses into biology. Along with graduate student Thomas Ellingham, Salick submitted a winning entry for the Cool Science Image contest, run through the website The Why Files.
The duo’s winning entry, an image of cellulose (plant-based) nano-fibers marked with a fluorescent stain, is pictured above. When dried, the fibers produce fascinating geometric, crystallized patterns that resemble a surreal, microscopic city skyline. The cellulose is derived from industrial waste by-products of energy production. The fibers are incredibly strong and are studied to explore incorporating them into materials for bio-sensors as well as materials with potential in automotive and aerospace applications.

The imaging work, in coordination with BIONATES researchers Wendy Crone, Lih-Sheng “Tom” Turng, Kris Saha and Randy Ashton as well as collaborators at the Morgridge Institute for Research, allows researchers to answer key questions while scanning cells and materials from the outside-in.
For instance, the group can assess whether cells in a given experiment are thriving or dying, whether they’re growing in ways intended and whether they’re sending signals that mimic health or disease.
“There are already several challenges involved with imaging biological samples, and these are all amplified when you expand these techniques into 3D live cell modeling,” Salick says. “To see more dynamically, we can genetically modify the cells and examine them under fluorescent light, which makes them more easily identifiable and allows us to see their biological mechanisms more clearly.”
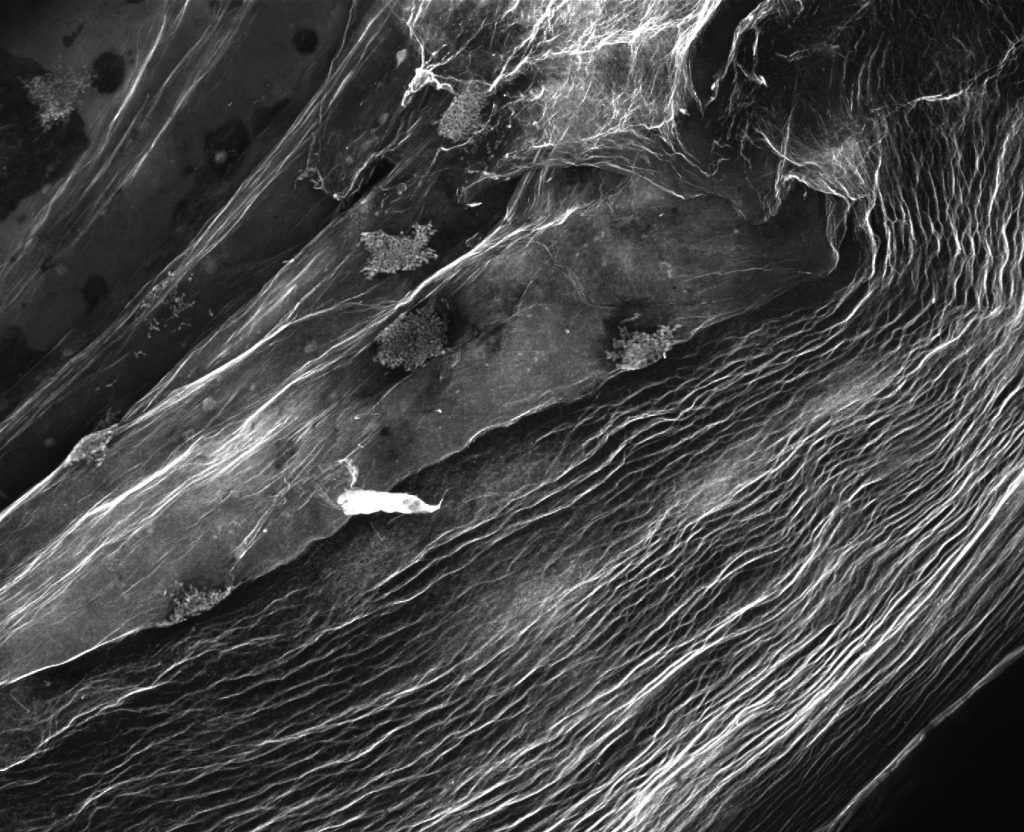
Pictured above: Bacteria that produce cellulose nano-fibers create porous 3D structures that can be used as scaffolding for tissue engineering and to manufacture high-strength materials. Resembling a satellite view of a foreign landscape, the image shows a fluorescent stained bacterial cellulose scaffold ready to be used for tissue culture. Image by Max Salick and Thomas Ellingham
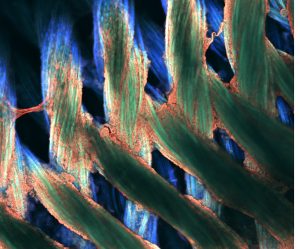
Pictured above: Silk tube structures with stem cells coating and growing on the surfaces. The Turng Lab, along with colleagues at the Morgridge Institute for Research, is experimenting with the tubes in efforts to recreate blood vessels. Image by Max Salick
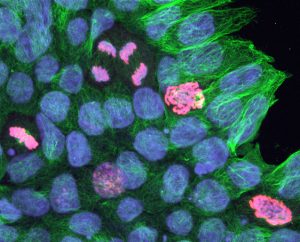
Pictured above: Neon-colored cells divide in a cell culture. Wendy Crone and graduate student Suehelay Acevedo are looking at how the mechanical properties of their surrounding might influence cells during division. The green stain marks centrosomes, which are one of the main mechanical components involved in cell division. The blue marks cell nuclei, and the red marks the DNA of dividing cells. Images by Max Salick
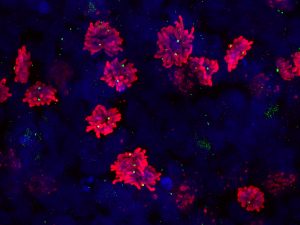
Pictured above: A scan of dividing cells with a 3D confocal scan, allowing scientists to see the orientation of the dividing cells — an important factor because the cells’ underlying substrate is direction-dependent. Images of this magnification also allows researchers to observe the number of centrioles (green dots) to determine if the cells are more predisposed toward developing abnormalities. Image by Max Salick
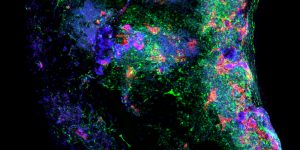
Pictured above: A blood vessel, taken as a cross-section of an engineered, cell-seeded tube. The red and green colors identify different cell types, which help researchers understand if these cells are capable of self-organizing to form the complex, layered morphology typical to tissue found in the human body. Image by Max Salick
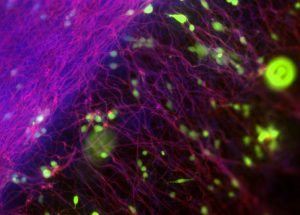
Pictured above: Electrospinning, a manufacturing technique commonly used for the production of fibrous, highly aligned biomaterials, allows the Turng Lab and Salick to create surfaces to culture fibroblasts (connective tissue) and understand early on whether materials are biocompatible with the human body. Image by Max Salick

You must be logged in to post a comment.