New Technology for Controlling Neural Tissue Manufacturing
Cells in the human body destined to become part of the central nervous system—like the brain and spinal cord, for example—do remarkable things. As the cells differentiate in vivo, they organize themselves into a single epithelial tube, the starting point from which the entire central nervous system develops. A paper published this week in eLife from Assistant Professor of Biomedical Engineering Randolph Ashton’s lab details a new method of reliably recreating this self-assembly in vitro.
Over the last 20 years, the promise of growing tissues from human pluripotent stem cells has opened up a world of possibilities in tissue engineering, transplant medicine, and drug development. But the transition from stem cells to functional tissue is difficult to control, limiting scientists’ ability to design dependable methods to efficiently create tissues and organoids. Developing tissues in vitro that function biomimetically—that is, similar to tissues developed in vivo—depends on emergence of a single organizing structure called an epithelial tube, but commonly referred to as a neural rosette because of its sunburst-like appearance. Cells orient and assemble around a rosette as the tissue develops. Current protocols, however, often yield numerous neural rosettes rather than a single morphogenesis center, resulting in tissues that do not behave like their in vivo counterparts.

Gavin Knight

Randolph Ashton
Gavin Knight, a postdoctoral associate in Ashton’s Stem Cell Bioprocessing and Regenerative Biomaterials Laboratory at the Wisconsin Institute for Discovery, is part of the team that discovered a new method to reproducibly control the early morphogenesis of human stem cell-derived tissues that will become neural organoids. “Cells that differentiate toward a neural lineage have the ability to self-organize,” he explains. “This phenomenon has a lot of promise for creating these tissues repeatably, but it’s very hard to do without a certain amount of engineering.”
That engineering involves identifying and optimizing biophysical parameters that constrain the physical size and geometry of the tissue as the cells differentiate toward their neural destinies. Ashton, Knight, and their collaborators have discovered that restricting the dimensions of tissues to particular sizes has a direct effect on the emergence of the single neural rosette tissue structure, the tell-tale organizing center critical to proper organoid development. The research team has zeroed in on dimensions for specific tissues that have a better than 80% success rate in inducing the development of a single neural rosette.
“If you wanted to reproducibly manufacture brain or spinal cord organoids by controlling every morphogenetic step, every developmental process, then use of this technology is an excellent place to start.” — Gavin Knight
“This is the starting point for engineering very well-controlled in vitro neural organoid morphogenesis,” says Knight, “and the ability to make advanced human tissues that are standardized.” Such control and standardization are key to building biomanufacturing protocols that result in tissues with biomimetic structures and function. With a repeatable and controlled process, scientists will be better able to grow tissues to better model and potentially treat neurodegenerative disorders and test drugs in human genetic backgrounds.
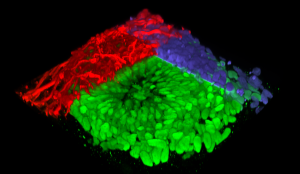
3D construction of a neural rosette organoid with colored immunolabeling
But even defining success would have been very difficult if not for the help of key collaborators. Narrowing the geometrical parameters for inducing neural rosette emergence required analysis of thousands of images to determine whether and how many cells had polarized, the sizes of developing rosettes, the orientations of adjacent cells, and other features. Traditional human examination would have seriously limited the number of samples that could be analyzed in the course of the project. Fortunately, the Ashton Lab’s location in the deliberately interdisciplinary Wisconsin Institute for Discovery afforded the team a unique opportunity to collaborate with computer science and machine learning experts to greatly increase their image analysis throughput.
Rebecca Willett, now a professor of statistics and computer science at the University of Chicago, was at the time a discovery fellow at WID. She and UW–Madison Professor of Electrical and Computer Engineering William Sethares helped Ashton’s group develop computational algorithms to segment and parameterize neural tissue images. Then, Assistant Professor of Consumer Science Lydia Ashton used those parameters to develop a machine-learning algorithm that could predict whether the tissue contain a single neural rosette structure. This collaborative effort allowed the Ashton lab to quickly and accurately determine whether their engineered tissues were growing properly. “This is an excellent example of next-generation tissue engineering and the requisite team work that can make it successful,” says Randolph Ashton.
“I don’t think we would have had that opportunity for that project without the physical proximity to Professor Willett and the collaborative assistance of Professors Sethares and Ashton,” adds Knight, who feels that the collaborative experience was a valuable part of his graduate training.
Knight, while recognizing that the platform he has helped to develop is only a first step, is enthusiastic about how it might advance the field of tissue engineering. “If you wanted to reproducibly manufacture brain or spinal cord organoids by controlling every morphogenetic step, every developmental process, then use of this technology is an excellent place to start.”
This work was made possible by grants from the National Science Foundation (CCF-1418976;IIS-1447449; 1651645), the National Institutes of Health (1 U54 AI117924-01), the Environmental Protection Agency (83573701), the National Institute of Neurological Disorders and Stroke (R21NS082618; R33NS082618), and the Burroughs Wellcome Fund (1014150).
–Nolan Lendved