New Technique Enables Versatile 3D Control Over Stem Cell-Derived Organoids
To bake a cake in a certain shape, you need the right mold. The same logic applies to creating organoids—collections of pluripotent stem cells that have formed tissues complex enough to mimic human organs.
But shape isn’t simply a cosmetic concern for organoids. It can have a direct effect on cellular diversity and organization, huge considerations when the whole point of the endeavor is to create tissues that replicate the ones in our bodies.
University of Wisconsin-Madison engineering professors Randolph Ashton and Lih-Sheng “Tom” Turng have partnered on a project to create hydrogel molds that will allow researchers to more precisely control the three-dimensional structures of organoids. They detailed their work in a recent paper in the journal Acta Biomaterialia.
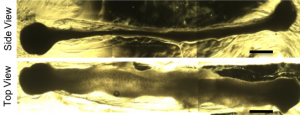
A brightfield microscope image shows organoids developing in a hydrogel after 16 days of culture. Image courtesy Ashton lab.
“At the scale of human development, which is micron to millimeters, there are not a lot of great technologies to control how tissues form at that level and in three dimensions,” says Ashton, an assistant professor of biomedical engineering and Wisconsin Institute for Discovery researcher.
Enter Turng, the Kuo K. and Cindy F. Wang Professor of Mechanical Engineering and Vilas Distinguished Achievement Professor. In recent years—and with the help of the collaborative research environment in the Wisconsin Institute for Discovery, he notes—Turng has applied his expertise in injection molding and polymer processing to the microscale, allowing him to work with Ashton to create a water-soluble plastic template.
Ashton’s lab then encapsulates the template in a hydrogel—Ashton likens it to a Jell-O mold—and the plastic dissolves, leaving a cavity that’s specifically shaped to drive anatomical tissue formation as the stem cells fill the cavity and differentiate down a specific organ pathway.
Essentially, they’re increasing the level of control in a process that’s generally governed by stem cell self-assembly into three-dimensional spheroids, which do not possess the natural geometrical constraints found in an embryo. Ashton says that method leads to an uncontrolled, spontaneous process of stem cell differentiation, giving rise to organoids with unnatural anatomies.

Carlos Marti-Figueroa, a PhD student in the lab of Biomedical Engineering Assistant Professor Randolph Ashton, works on injecting stem cells into alginate hydrogels. Photo: Renee Meiller.
“We’re trying to harness the ability of the stem cells to self-form a lot of these structures that we as scientists cannot form ourselves,” says Carlos Marti-Figueroa, a PhD student in Ashton’s lab and one of the paper’s first authors. “A lot of the cellular diversity that occurs has to do with the self-organization capabilities of the cells.”
Ashton and his research group are particularly interested in using the technique to create spinal cord organoids, which they could use to better understand development of the spinal cord, diseases that affect it (such as amyotrophic lateral sclerosis and spinal muscular atrophy), and neurotoxic effects of pharmaceuticals and environmental toxins.
The research is part of the National Science Foundation CAREER Award he received in 2017, which also includes an outreach exhibit that will soon launch on the first floor of the Discovery Building.
Jason McNulty, a PhD graduate in mechanical engineering, shared first authorship with Marti-Figueroa. Other authors include Frank Seipel, an undergraduate researcher in Ashton’s lab; Joshua Plantz, a former Ashton lab undergraduate researcher who died in January 2018; Thomas Ellingham, a PhD graduate in mechanical engineering; Lukas Duddleston, a PhD student in materials science; Sebastian Goris, a PhD graduate in mechanical engineering; Benjamin Cox, a research assistant in the Department of Medical Physics; and Professor Tim Osswald, the Consolidated Papers Foundation Chair sponsored by the Mead Witter Foundation in the Department of Mechanical Engineering.
This story originally appeared on engr.wisc.edu. Author: Tom Ziemer