Scientists Seek to Improve Quality Control for Genome Editing Therapies in the Eye
As gene editing therapies for macular degeneration and other visual disorders work their way into clinical trials, the University of Wisconsin–Madison is on the forefront of research into making sure they are safe and effective.
Nine years ago, David Gamm, M.D., Ph.D., Director of the McPherson Eye Research Institute (MERI) and associate professor of ophthalmology and visual sciences, grew the first early retinal structures from human induced pluripotent stem cells in the lab, reporting in Proceedings of the National Academy of Sciences of the United States of America.

Krishanu Saha
Today, a team of scientists in the School of Medicine and Public Health and the College of Engineering led by Dr. Krishanu Saha, Ph.D., assistant professor of biomedical engineering at the Wisconsin Institute for Discovery (WID), join the National Institutes of Health’s Somatic Cell Genome Editing Consortium after receiving a major collaborative award (U01). The investigators aim to develop quality control methods for improving genome editing therapies in the eye. Granted through the National Center for Advancing Translational Sciences, the award builds up on a Round Five UW2020 award to the UW-Madison Graduate School.
In addition to Saha and Gamm, collaborators include Sushmita Roy, Ph.D., associate professor of biostatistics and medical informatics at WID, Melissa Skala, Ph.D., associate professor of biomedical engineering and investigator at the Morgridge Institute for Research, Rupa Sridharan, Ph.D., assistant professor of cell and regenerative biology at WID, and Bikash Pattnaik, Ph.D., assistant professor of pediatrics at MERI. The grant includes additional members from MERI, which fosters multi-disciplinary collaborations to address unmet therapeutic needs and combat blinding disorders.
“We aim to create human retinal tissues from pluripotent stem cells to serve as the platform for testing gene editing effects,” says Gamm, whose team created and advanced this technology in his lab at the Waisman Center.
“The impact of this work could be broad, with the potential to advance the development of genome editors administered to any tissue to treat a variety of diseases.” — Kris Saha
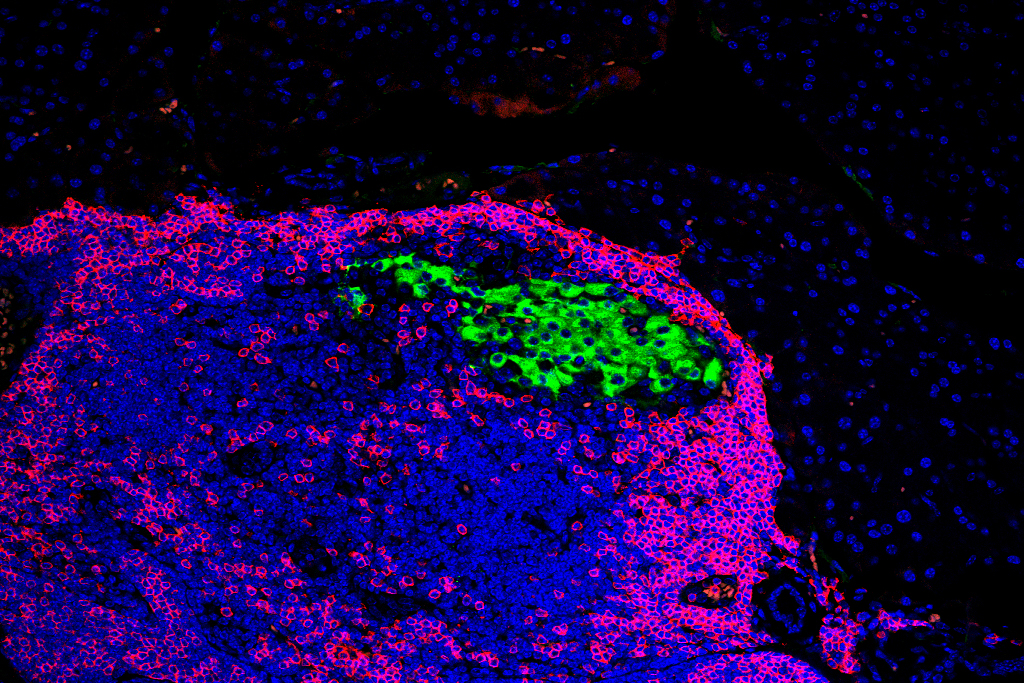
The researchers then plan to use specialized imaging and gene sequencing methods to define biomarkers for any adverse events after delivery of CRISPR-Cas9 genome editors to the tissues. They will focus on examining rod and cone photoreceptors using 3D optic vesicle structures made from the stem cells.
If successful, their work could significantly advance therapeutic genome editing in the eye and extend to more therapies involving more complex cultures and tissues.

Sushmita Roy
Rupa Sridharan
“Ultimately, our imaging technologies could be used for quality control so that we’re sure edited cells are functional and not capable of initiating disease in patients,” adds Skala.
Genome editing holds great promise in both basic and translational research. Unfortunately, some formulations of these therapies may produce unwanted adverse effects, such as an inflammatory response and even cancer.
“Screening for adverse events is essential for the development of safe genome editing therapies,” Saha says. “By using 3D eye-like structures made from stem cells, our approach is groundbreaking in attempting to track these events in very complex tissues. If our approaches work well within these retinal structures, the impact of this work could be broad, with the potential to advance the development of genome editors administered to any tissue to treat a variety of diseases.”
UW2020 awards stimulate and support highly innovative and groundbreaking research at the University of Wisconsin–Madison. The Wisconsin Alumni Research Foundation (WARF) underwrites the awards, which include combined funding from the National Institutes of Health Common Fund and other sources.
This story originally appeared on research.wisc.edu. Author: Jordana Lenon.