While studying the three-member model microbial community, nicknamed The Hitchhikers of the Rhizosphere (THOR), researchers from professor of plant pathology and director of the Wisconsin Institute for Discovery Jo Handelsman and professor of biomedical engineering and Discovery Fellow David Beebe’s labs noticed cells moving in unexpected, unique ways under the microscope. They dubbed this new phenomenon “zorbs” in a recent Nature Communications publication, for the cells’ similarity to the plastic toys people run around in. Bacterial zorbs are a story of discovery in unanticipated places and the importance of multidisciplinary collaboration.
Bacteria exist in the world as individual cells, in communities with other species, and sometimes as biofilms, which are clusters of cells and extracellular materials, such as sugars, lipids, proteins, and nucleic acids. Biofilms are structures that are typically stationary, attach to surfaces, protect cells against threats, and are found in many different environments, including the soil.
THOR is comprised of three plant-root associated species: Pseudomonas koreensis, Bacillus subtilis, and Flavobacterium johnsoniae. When grown together, these species form biofilms. Research on interactions among THOR members, including biofilm formation, previously showed P. koreensis as the largest biofilm producer of the three species when cultured together, but new technology exposed different results.
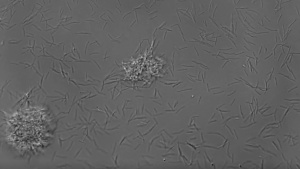
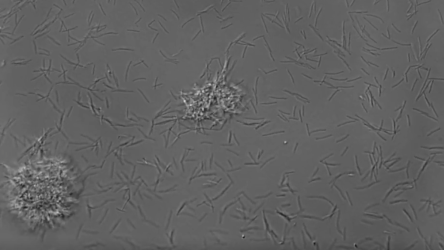
Microfluidic tools allow for precise manipulation of fluids and visualization of fluidic processes at a small scale. Beebe lab scientist Chao Li recently developed an under-oil open microfluidic system (UOMS) with various biological and biomedical applications. One such application was studying biofilm formation of THOR, as UOMS can be used to examine cells across space and time. Using this system, the team saw that F. johnsoniae produced more biofilm than P. koreensis, and even more shocking, these F. johnsoniae microcolonies were motile.
The rod-shaped soil bacterium, F. johnsoniae, appeared to glide across the surface lengthwise, then stand upright, assemble into microcolonies, merge with other microcolonies, and eventually disperse. This collective movement did resemble other organisms like Neisseria which use hair-like pili to move and merge. However, individual F. johnsoniae cells lack pili and instead utilize another form of movement called gliding motility, where molecules on the cell membrane attach to a surface and propel the cell along. The researchers named these unique microcolonies “zorbs” for their resemblance to the large, plastic orbs humans bump, roll, and run around in.
Together, biochemical and genetic approaches helped answer questions as to whether the self-propelled social movement required energy. As expected, the zorbs assembled using energy, but the questions of how and why this occurred remained.
The Handelsman and Beebe labs then teamed up with associate professor of mechanical engineering Wenxiao Pan’s lab which revealed some insight. Former Pan lab postdoc Wei Hu developed a new computational method to identify the force required to propel both individual cells and microcolonies in the liquid environment, suggesting cells act together to generate the force needed to move the zorbs.
Collaboration across research groups made this unexpected publication a success. “Without interdisciplinary collaboration, this paper wouldn’t have been written. It draws on so much deep expertise,” former Handelsman lab postdoc Amanda Hurley says. “And the research is just more exciting since we can answer more questions when it’s multidisciplinary.”
The exact reasons why F. johnsoniae forms zorbs are still unknown, but the authors hypothesize the collective movement is related to biofilm dynamics, adaptation, or moving nutrients between organisms. The researchers already have intriguing preliminary data on how zorbs interact with B. subtilis, another member of THOR, but have yet to explore zorb function in their natural environment on plant roots. This work raises many future questions and serves as a reminder to be open to surprises.
“This is a story of discoveries happening in unexpected ways and following what the experiment is telling you, rather than how you had it envisioned in your mind,” says Hurley.
This work was supported by the NSF, NIH, EPA, American Cancer Society, US Army Research Laboratory and the US Army Research Office, and USDA.
You must be logged in to post a comment.