
Scientists at the Wisconsin Institute for Discovery (WID) at the University of Wisconsin–Madison have identified a surprising reason some microbial communities in the rhizosphere—the soil region influenced by plant roots—flourish over others: stickiness. A new study reveals how adhesive traits significantly contribute to a bacterium’s success at colonizing surfaces in communities. That leads to an important question: what exactly makes them sticky?
Despite its widespread presence in soil and rhizosphere communities, the success factors for Flavobacterium species were largely unknown until now. Critical for plant health, Flavobacterium promotes plant nutrient uptake, increases stress tolerance, and disease suppression by outcompeting or inhibiting the growth of harmful pathogens. Learning why this bacterium is successful at colonizing surfaces, particularly in the rhizosphere, could pave the way for advances in agriculture to enhance soil health and natural plant growth.
This led the team to investigate Flavobacterium johnsoniae, one species within the genus Flavobacterium, to understand why it is sticky, as its presence is crucial to the soil region called the rhizosphere. This region serves as a bustling hub for bacterial activity, and is a vibrant ecosystem critical for plant health, facilitating nutrient cycling, disease control, and symbiotic relationships between roots and microbes, including bacteria and fungi. The rhizosphere is influenced by root secretions and associated soil microorganisms. This suggests that the absence of F. johnsoniae might affect plant health, potentially influencing growth, yield, and overall ecosystem stability to some degree.
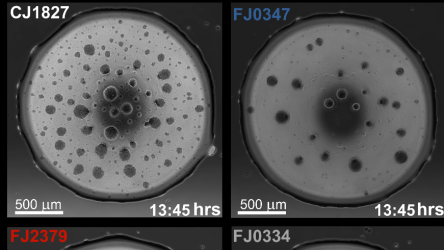
Using a model microbial community created by the Jo Handelsman Lab and dubbed The Hitchhikers of the Rhizosphere (THOR), researchers gained insight into the bacterium’s survival strategies. They explored how forming a stable community affects one such denizen in the Flavobacterium species, F. johnsoniae. The study identified 25 genes essential for surface colonization, which is crucial for F. johnsoniae’s survival in the dense soil microbial ecosystem. The widespread presence of F. johnsoniae is the bacterial equivalent of finding a perfect neighborhood in a crowded city.

“In our study, we found that the bacterium Flavobacterium johnsoniae’s ability to colonize surfaces, such as sand, enhances its survival within microbial communities,” says first author Shruthi Magesh, a graduate student in the Jo Handelsman lab of the Microbiology Doctoral Training Program at WID.
“We identified unique genes in these bacteria that equip them with ‘superpowers’ for clinging to surfaces and creating slimy layers,” says Magesh. While investigating the deletion of some colonization genes, the study revealed a genetic connection between the formation of stationary and motile biofilms in F. johnsoniae, illustrating biofilms’ role as bacterial fortresses offering shelter and resources. A motile biofilm refers to a community of microorganisms that can move as a collective unit across a surface. Unlike traditional biofilms, which are typically sessile (non-moving) and adhere strongly to surfaces, motile biofilms can relocate in response to environmental conditions. The study identified several genes in F. johnsoniae that are crucial for surface colonization and demonstrate their significance in both solitary and community settings.
Investigating the deletion of genes sheds light on the relationship between colonization, biofilm formation, and fitness, offering crucial insights for researchers striving to understand microbial communities more deeply. The next steps include investigating how these genes change the behavior of microbial communities and affect their interactions with plants in the soil around the roots. Learning about the formation and function of these bacterial biofilms could be key to enhancing soil health, ultimately leading to healthier plants and more robust ecosystems—unearthing the secrets of stickiness in the process.
-Laura RedEagle
This study was supported by the U.S. Army Research Laboratory and the U.S. Army Research Office under contract/grant W911NF1910269, the Vilas Trust, the Office of the Vice Chancellor for Research and Graduate Education at the University of Wisconsin-Madison, the University of Wisconsin Carbone Cancer Center Support Grant NIH P30CA014520, and NIH R01AI59940. A.I.H. was supported by USDA NIFA Grant 2019-2018-08058 (Accession no. 1019190). J.F.N. was supported by an NHGRI training grant from the Genomic Sciences Training Program 5T32HG002760. M.G.C. was supported by USDA NIFA Grant 2020-67012-31772 (Accession no. 1022881). C.L. was supported by NSF EFRI-1136903-EFRI-MKS, NIH R01 CA247479, NIH R01 AI154940, NIH R01 EB010039, NIH R01 CA185251, NIH R01 CA186134, NIH R01 CA181648, NIH R01AI132627, NIH P30CA014520, EPA H-MAP 83573701, and American Cancer Society IRG-15-213-51.
You must be logged in to post a comment.